Entropy, Equilibrium and Climate Modelling.
I don’t know about you, but I still have many questions about the fundamental science behind the whole Climate Change “debate”. “The Science” is far from settled in my mind.
There are many scientific questions that still nag me.
I awoke this morning thinking about entropy and the equilibrium of gases. This is a topic that I have not much considered since Grade 13 Chemistry in my 1968-69 school year, but it seems like a relevant subject to present to you for consideration in the Climate Change “debate”.
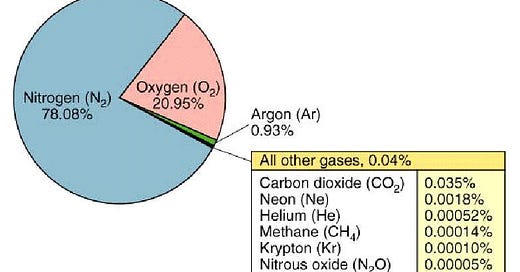
The attached image shows that the earth’s atmosphere is composed of various gases: Nitrogen (~78%), Oxygen (~21%), Argon (~0.9%), CO2 (0.04%), methane (O.00014%) and other trace gases. Of these, only CO2 and methane are greenhouse gases (they vibrate to release heat when subjected to UV radiation - sunlight) .
I have linked papers on the Four Laws of Thermodynamics and the Conservation of Energy for your reference.
My questions from this morning are:
Are these gases at uniform concentration everywhere and always within the earth’s atmosphere? If Yes, how do we know - do we rely on theory generated under laboratory conditions or has this theory been tested globally? If No, then what are the external factors that can affect equilibrium and in what way can they do so?
Gas volumes (V) are known to change with T (temperature) and P (pressure). Do we know on a planetary scale when and if the entropy of gases is affected by V, P and T? For example, what if I were to measure CO2 concentrations at ocean level in Amsterdam on a day when atmospheric P measurement was high and T was +30 degrees Celsius (mid-summer), and compared these data with CO2 measurements from Anchorage, Alaska in mid-winter when P is low and T is -30 degrees Celsius. Could I expect the identical CO2 concentrations (assuming the the measuring devices are in perfect calibration with each other)?
If anyone reading this can answer these questions, I would appreciate it.
My interest is primarily in the accuracy and reliability of the CO2 (and temperature) datasets that have been used by scientists who work with computer models to forecast rises in global temperatures from now until the year 2100. I am also interested in how these modellers have controlled for the dozens of other variables that can affect the two main variables of interest: Global CO2 and Global Temperatures.
For the rest of you who are not interested in this topic, I apologize for “geeking out” on you today.



Very good questions and we could add many more.
Not only about CO2 variations in different climates, but also the very measurability of the effect.
Everything about global warming is a theory, not supported by any measurable empirical evidence.
A Reply.
A friend of mine graduated with a Physics degree about the same year. He was kind enough to provide the following comment to to questions:
======
The short answer to your first question is both yes and no. Earth's atmosphere is a solution and
just like a glass of muddy water, if it was left to stand completely still then it would
separate out. However, Earth's rotation acts like a gigantic mix master and keeps things mixed
pretty evenly. There are also things such as the differential heating of the Earth by the Sun as it
rotates. At very high altitudes, other effects begin to come into play and mix actually changes. How
do we know this? We've been measuring the atmosphere and the gases it contains since the early
1800's and we are still measuring it thousands of times a day throughout the world although the
instruments used are much more sophisticated. There are literally (in the true definition of
literal) hundreds if not thousands of satellites in orbit that cover every sq centimetre of the Earth
and are continually making these kinds of measurements. There are measured and understood differences in these gas ratios throughout the world. For example, the ratios are slightly different over the poles but I do not believe these differences are great or significant in the scheme of the whole
Earth.
Entropy is not a major influencer in the overall scheme primarily because the Earth is not a closed
system.
As for equilibrium, that depends on what you're really asking. At a molecular leave, the atmosphere
is most definitely not in equilibrium.
As for question 2, entropy is not the factor to consider here for the reasons given above. I've never really investigated this but I would expect that temperature and pressure would have an impact on the gas ratios in a open system such as the Earth but that is just my guess. I would also expect these not to be very significant and that the ratios would mostly hold. This is of course would only be relevant if you measure the ratios by volume.
If the ratios are measured by parts per million or some similar method
that is counting actual numbers molecules then I would expect the ratios to remain exactly the same
due purely to the conservation of mass law. The biggest influencer to keep in mind here is the
Earth's mix master effect which keeps everything pretty much equally stirred. I also would strongly
expect that all of the equations used to model climate are based on a parts per million type
measurement and not a volume ratio. The chemical reactions that are going on in the environment are after-all happening at a molecular level.